An Indirect Potentiometric Determination of Anions (or Cations) Using a Precipitation Reaction and a Standard Addition Method Not Requiring Adjustment of an Ionic Strength of Sample†
Norihisa ISHIKAWA*, Hiromoto SUGITANI, Ming-Jie LI†† and Hiroshi MATSUSHITA
Department of Applied Chemistry, College of Engineering, Chubu University; 1200 Matsumoto-cho, Kasugai-shi 487-8501 Japan
†† Department of Chemistry, Yian-Bian University; 105 Gong-Yuan-Lu, Yian-Ji-shi 133002 China
An indirect potentiometric determination of anions (or cations) using a precipitation reaction and a standard addition method, in which the adjustment of the ionic strength of a sample is not required, is proposed.
A certain volume (Vr) of the solution (reactant) containing the precipitant cation A of a known concentration cr is added to the sample solution of volume V containing the analyte anion B of a concentration cx. If the composition of formed precipitate is AmBn, the condition of crmcxV/(nVr) needs to be sat isfied. After an A-selective electrode and a reference electrode are immersed to the sample added the reactant, this solution is titrated with the solution (standard-1) containing A of a known concentration (cs1), where the added volumes and the final added volume of the standard-1 are denoted with vs1 and vs10 respectively. The electromotive forces (E1) which correspond to added volumes (vs1) of the standard-1 are measured. Subsequently the same sample solution of volume V and the reactant of volume Vr are added to titrated sample. This solution is titrated again with the solution (standard-2) containing A of a known concentration cs2 (>cs1) and having the same ionic strength as that of the standard-1. The electromotive forces (E2) which correspond to added volumes (vs2) of standard-2 are measured.
If E1 and E2 which correspond to vs1 and vs2 that satisfy a condition of vs2=2vs1−vs10 are read off from two titration curves, the following equation is held concerning with the concentration cx of B:
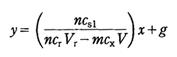
where y=10ΔE/S, x = vs1{(cs2/cs1)−y}, ΔE=E2−E1, and S and g are the response slope of the A-selective electrode and a constant respectively. This cx is determined from the slope of linear plots of y vs. x.
By using a silver ion as the precipitant and a silver ion-selective electrode as an indicator electrode, concentrations of a hexacyanoferrate(II) ranging from 1×10−2 to 5×10−4 mol dm−3 in the samples of various ionic strengths were determined with an error of less than approximately ±1 % and a relative standard deviation of less than 1 %.
† An Indirect Potentiometric Determination Not Requiring Adjustment of an Ionic Strength of Sample. Part 2.
[Contents (In Japanese) ]
[Contents (In English) ]