Sorption Equilibria of Amino Acids by Anion Exchange Resins
Masahiro TAKAHASHI
Department of Chemistry and Biochemistry, Suzuka National College of Technology; Shiroko-cho, Suzuka-shi 510-0294 Japan
Studies have been conducted on the sorption equilibria of seven kinds of amino acids by anion exchange resins of strong basic type (Amberlite IRA-401B) and weak basic type (Duolite A-7).
The sorption equilibria of amino acids (A−) and hydroxide ion on anion exchange resin of Cl type can be expressed by following equations.
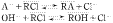
The sorption equilibrium constants of amino acids, KA, were 14.3 (Trp), 4.76 (Phe), 1.52 (Leu), 0.909 (Ala), 0.833 (Gly), 1.00 (Glu), 0.909 (Asp) for Amberlite IRA-401B resin, and 3.57 (Trp), 2.00 (Phe), 0.714 (Leu), 2.50 (Ala), 0.714 (Gly), 0.357 (Glu), 0.333 (Asp) for Duolite A-7 resin, respectively. The sorption equilibrium constants of hydroxide ion, KOH, were 4.00 for Amberlite IRA-401B resin and 1.10 for Duolite A-7 resin, respectively. The highest KA value was obtained for Trp, being larger than the value for Asp by a factor of about 16 for Amberlite IRA-401B and about 11 for Duolite A-7 resin.
It was found that the sorption equilibrium constants of amino acids increase with increasing hydrophobicity except sorption of Ala and Gly for Duolite A-7 resin.
[Contents (In Japanese) ]
[Contents (In English) ]