Kumiko MIKI*, Isao YAMAKAWA and Toyohiko NAKAJIMA
Department of Liberal Arts and Basic Science, College of Industrial Technology, Nihon University; 2-11-1 Shinei-cho, Narashino-shi 275-8576 Japan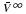 , were determined. The molar volumes, VB, was calculated from Eq. 4. VB is the value that represents volume of solutes with interacting water.
, were determined. The molar volumes, VB, was calculated from Eq. 4. VB is the value that represents volume of solutes with interacting water.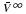 with -H→-CH3 substitution were not considerable in the case of the straight chain or in the case of the branched chain (Scheme 2). However, the change in VB with -H→-CH3 substitution in the case of the the branched chain was much larger than that in the case of the straight chain (Scheme 1).
with -H→-CH3 substitution were not considerable in the case of the straight chain or in the case of the branched chain (Scheme 2). However, the change in VB with -H→-CH3 substitution in the case of the the branched chain was much larger than that in the case of the straight chain (Scheme 1).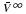 with -H→-CH3 substitution in the case of the branched chain was formed stronger than that in the case of the straight chain (Scheme 3 and Scheme 4). This fact was supported by the values of hydration entropy (Scheme 5).
with -H→-CH3 substitution in the case of the branched chain was formed stronger than that in the case of the straight chain (Scheme 3 and Scheme 4). This fact was supported by the values of hydration entropy (Scheme 5).