Breaking Benzene by a Molecular Titanium Hydride Cluster
The hexagonal aromatic benzene ring is a ubiquitous structure motif, widely existing in natural resources such as petroleum and biomass. The cleavage of a benzene ring is of great interest and importance, as this transformation can be used to produce fuels and other important chemicals from natural resources. However, the carbon skeleton of benzene is very stable. In chemical industry, the cleavage of a benzene ring is performed at high temperatures (>500℃) on solid catalysts. This process is quite complicated and usually yields a mixture of several products.
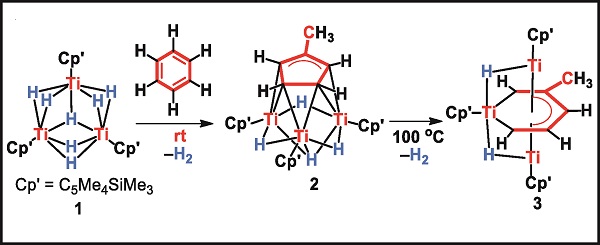
In our recent studies on multinuclear rare-earth and transition metal hydride complexes,1 we found that a trinuclear titanium heptahydride complex (1) could break and partially hydrogenate N2 at ambient temperature and pressure.2 When this Titanium hydride cluster was allowed to react with benzene, the carbon-carbon bond cleavage and rearrangement of benzene took place at room temperature, quantitatively yielding a ring-contraction product [MeC5H4] (2).3 In this transformation, a robust C−C bond of benzene is cleaved, together with formation of a new C−C bond. Upon heating at 100 °C, a Ti atom was inserted into a C-C bond of the MeC5H4 ring in 2 to give a titanacycle product (3). Other aromatic compounds such as toluene and pyridine could also be cleaved in a similar fashion. In all of these transformations, the cooperation of multiple titanium hydrides has played a critically important role. These studies may facilitate the design of new catalysts for the transformation and functionalization of inactive aromatics.
1) M. Nishiura, Z. Hou, Nature Chem. 2010, 2, 257.
2) T. Shima, S. Hu, G. Luo, X. Kang, Y. Luo, Z. Hou, Science 2013, 340, 1549.
3) S. Hu, T. Shima, Z. Hou, Nature 2014, 512, 413.
Zhaomin Hou, RIKEN Center for Sustainable Resource science