Synthesis of Organic Nitrogen Compounds from Biomass
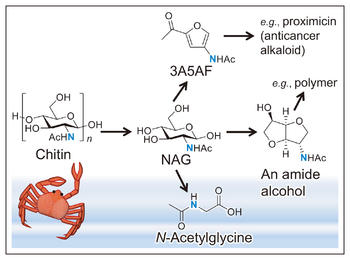
Chitin, a polymer of N-acetylglucosamine (NAG) linked by b-1,4-glycosidic bonds, is a prominent marine biomass. The characteristics of chitin, made almost entirely of repeating nitrogen-containing units, has recently attracted the attention of researches in the field of green chemistry who seek an alternative resource for producing organic nitrogen compounds1).
The crucial first step in chitin utilization is hydrolysis of chitin to NAG. Industrial processes use a large amount of concentrated hydrochloric acid and enzymes, or rely on re-acetylation of glucosamine after complete hydrolysis of chitin. However, the inefficiency of these methods is a major challenge. We have proposed mechanocatalytic hydrolysis of chitin which uses mechanical force of ball-milling and a catalytic amount of acid2). In this method, the amount of acid can be greatly decreased in comparison to current industrial processes. Moreover, the mechanocatalytic hydrolysis ensures retention of the amide groups.
Downstream conversion of NAG obtained from mechanocatalysis can produce 3-acetamido-5-acetylfuran (3A5AF), a source for aromatic compounds and medicines3-5); an amide alcohol with a condensed five-membered ring for polymer synthesis6); and N-acetylglycine, a derivative of a standard amino acid7). These compounds are only produced through chitin processing, and therefore this research field is experiencing a new direction which departs from the widely studied conversion of biomass containing only C, H and O atoms such as cellulose, starch and vegetable oils.
 1) N. Yan et al., Nature 2015, 524, 155.
1) N. Yan et al., Nature 2015, 524, 155.
2) M. Yabushita et al., ChemSusChem 2015, 8, 3760.
3) Y. Liu et al., ChemPhysChem 2014, 15, 2723.
4) M. Osada et al., Fuel Proc. Technol. 2019, 195, 106154.
5) A. D. Sadiq et al., ChemSusChem 2018, 11, 532.
6) T. Sagawa et al., ACS Sustainable Chem. Eng. 2019, 7, 14883.
7) K. Techikawara et al., ACS Sustainable Chem. Eng. 2018, 6, 12411.
KOBAYASHI Hirokazu, Institute for Catalysis, Hokkaido University